(1) INNATE IMMUNITY SIGNALS IN REGULATING MASSIVE INFECTED CELL EXTRUSION
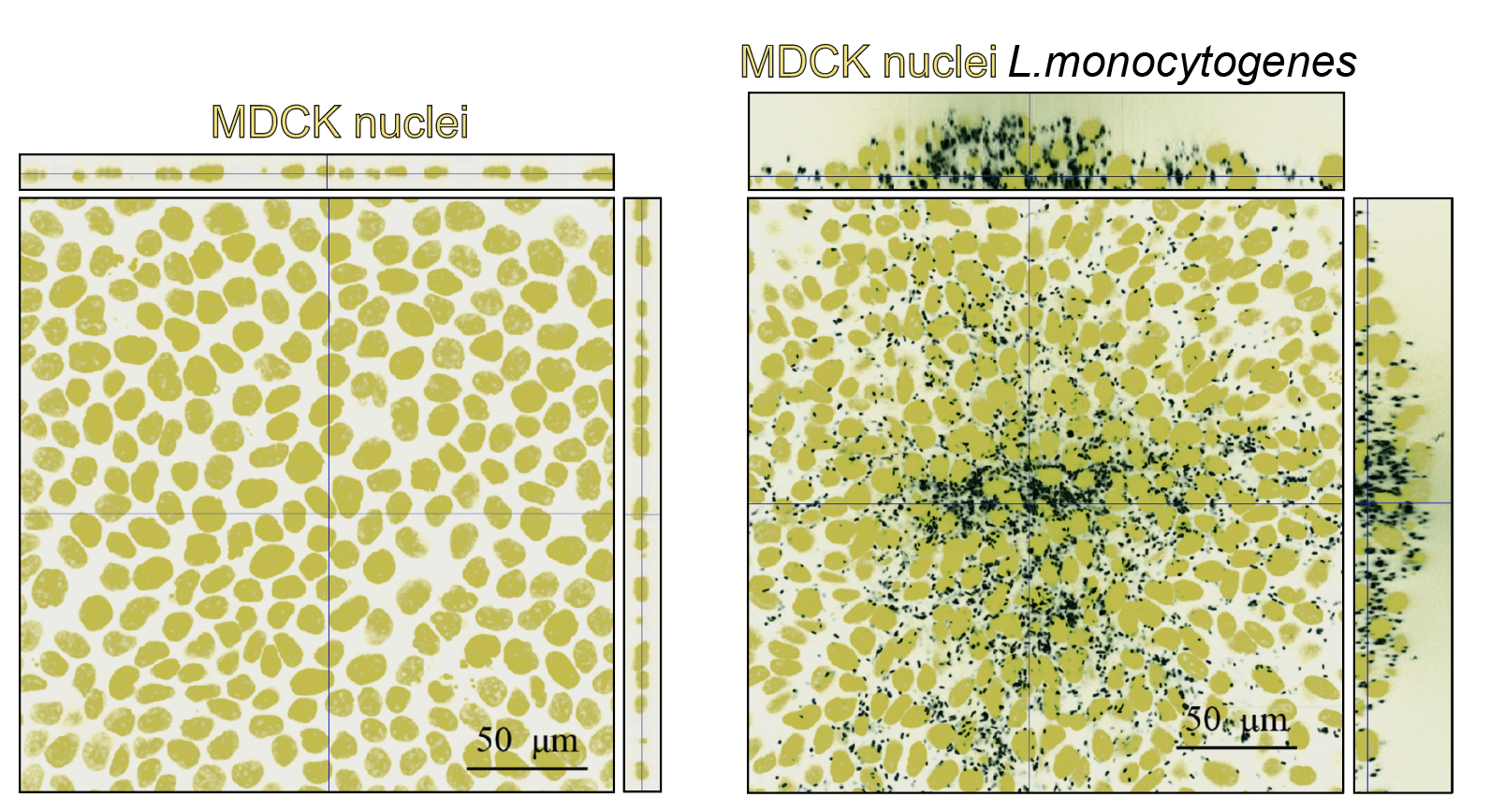
During infection with the food-borne intracellular bacterial pathogen L. monocytogenes, large infected domains in epithelial cell monolayers are forced to extrude to form three-dimensional mounds due to the collective and cooperative onslaught by their uninfected neighbors. This mechanical competition between uninfected and bacterially-infected cells limits pathogen dissemination through the epithelium (Bastounis et al, Dev Cell, 2021). We investigate how signaling and cytoskeletal dynamics correlate with changes in host cell mechanics during bacterial intercellular spread. We also explore the contribution of extracellular mechanics (extracellular matrix mechanics, shear flow and mechanical strains) in promoting or obstructing this battle for the benefit of the host.
Orthogonal views of uninfected (left) or L. monocytogenes-infected MDCK epithelial cells (right) at 24 h post-infection. Host cell nuclei: yellow, L. monocytogenes: black. Note the infection mound with cells piled up on the right.
(2) EXTRACELLULAR MATRIX (ECM) STIFFNESS AND FLUID SHEAR DURING INTRACELLULAR BACTERIAL DISSEMINATION
Endothelial cells (ECs) line the inner lumen of our vessels and act as gatekeepers protecting us from a variety of insults, including pathogens. ECs are constantly exposed to shear stresses due to blood flow and are experiencing alterations in the stiffness of the ECM on which they reside. Changes in both these mechanical cues contribute to multiple pathologies including atherosclerosis, and often correlate with increased susceptibility to bacterial infection. However, most clinical studies on these correlations lack causality and are rather observational in nature. We have adapted a multi-well impinging flow jet to impart varying shear stresses and gradients into ECs in culture residing on varying stiffness ECM and are investigating how that impacts direct adhesion and uptake of pathogenic intracellular bacteria, as well as their ability to spread from cell to cell (Bastounis et al, MBoC, 2018; Surya et al, MBoC, 2019; Bastounis et al, Sci Rep, 2019). Our goal is to discover how shear flows impact host EC mechanotransduction and tension, thus facilitating or obstructing intracellular bacterial pathogen dissemination (Lamason, Bastounis et al, Cell, 2016; Faralla*, Bastounis*, PLoS Pathog, 2018).
Sketch depicting different techniques we use to probe host cell mechanics during infection by intracellular bacterial pathogens.
Left: Cartoon depicting heterotypic interactions of L. monocytogenes-infected macrophages with endothelial cells. Right: Phase contrast images of uninfected endothelial cells (controls) or endothelial cells exposed to L. monocytogenes-infected macrophages.
(3) HOST CELL MECHANICS IN HETEROTYPIC INTERACTIONS OF INFECTED MACROPHAGES WITH ENDOTHELIAL CELLS
One way that intracellular bacterial pathogens like L. monocytogenes spread systemically in vivo is by being carried from tissue to tissue within immune cells. Thus, it is critical to understand how infected immune cells transfer pathogens into endothelial cells or transmigrate through them. By co-culturing endothelial cells residing on varying stiffness matrices with infected macrophages, we aim to explore the roles (1) of the mechanics of “donor macrophage” versus “recipient endothelial cell” during heterotypic bacterial transfer and (2) of the tensional forces of endothelial cells (that can be thought of as a proxy of the endothelial cell barrier integrity) in regulating infected-macrophage transmigration though the endothelium.